The certification journey of the EpiShuttle
What makes a high-quality patient isolation unit?
A thorough and comprehensive compliance and certification strategy is at the backbone of high-quality medical devices, and the EpiShuttle is no exception. When it comes to transporting and isolating patients, safety and effectiveness can only be achieved through rigorous certification and strict regulatory compliance, ensuring that every aspect of a device meets the highest standards. Only then can a medical device truly be trusted to protect both patients and healthcare professionals in the most critical moments.
The EpiShuttle’s compliance and certification journey spans over nine years of thorough testing, revision and enhancement. As a transport isolation unit designed to also enable medical care, the EpiShuttle certification strategy needed to prioritize patient and provider safety while also ensuring vehicle safety and compatibility. That required a strategy focusing on several fronts: CE certification, tests for leakage and validation of reprocessing proceeded parallel with flammability, rapid decompression, crash, vibration and EMC testing to name a few.
Our regulatory milestones
To give you a clearer picture of this journey, we’ve created a timeline that captures some of the most critical milestones (text description below the infographic).
Yet, our work is far from finished. EpiGuard constantly strives to raise the bar, continually testing and improving both our company and our products to meet the ever-evolving safety and operational standards demanded for modern medical devices. While the EpiShuttle has come a long way, we believe this is just the beginning of an ongoing journey towards excellence. We are dedicated to pushing boundaries and setting new standards for quality and safety.
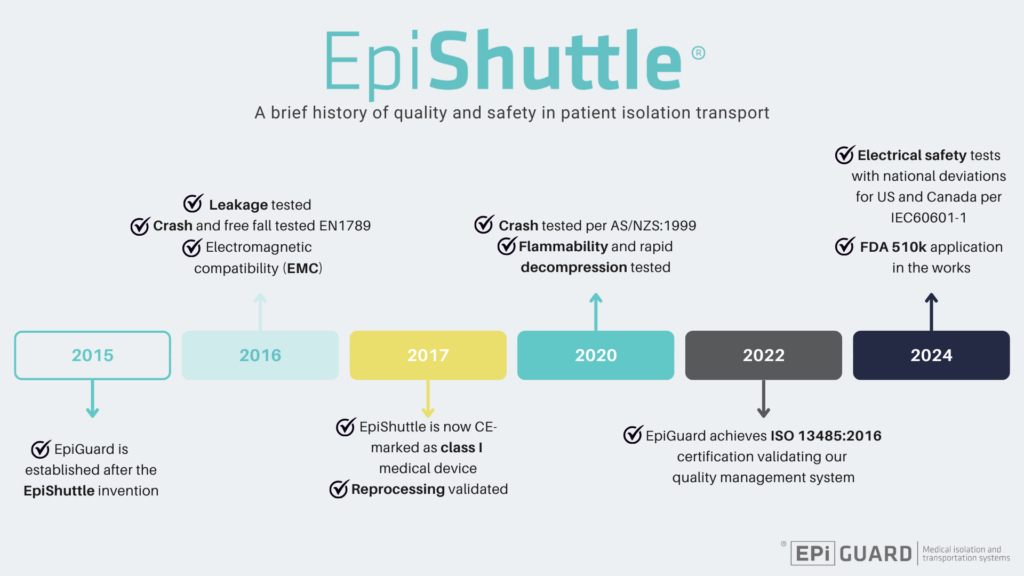
Our key milestones :
2015:
After the invention of the EpiShuttle, EpiGuard AS is established.
2016:
The EpiShuttle successfully goes through leakage testing from Norwegian Defence Research Establishment FFI.
Crash and free fall testing is successfully performed according to EN 1789:2020.
Why is this important?
The CEN 1789 European Standard specifies requirements for the design, testing, performance and equipping of road ambulances used for the transport and care of patients. The EpiShuttle is especially designed for use in emergency vehicles for transport of patients, including road ambulances. Without it, EpiShuttle wouldn’t be safe to load in a patient transport vehicle. Making sure that the EpiShuttle meets the standards for road safety was key in our technical and design processes and implemented in our robust base with integrated L-tracks, together with 5-point harness and patient belting anchored to it.
Electromagnetic compatibility (EMC) as per CB, EIC 60601-1-2 is certified.
2017:
The EpiShuttle is CE-mark as class I medical device.
Reprocessing procedures are validated.
Why is this important?
The EpiShuttle users are often required to respond to situations involving high-risk pathogens and contaminants. As manufacturers, we provide users validated and well-documented guidance on how to safely reprocess our products. See an example of our validation processes at this link.
Start of commercialization of the EpiShuttle.
2020:
Impact testing according to AS/NZS:1999 is performed and passed.
The EpiShuttle is cleared for flammability and rapid decompression testing according to CS 25.853 Appendix F.
Why is this important?
Passing flammability tests is a necessity for aircraft safety. Rapid decompression guarantees equipment functionality under loss of pressurization at max operating altitude or emergency descent. The EpiShuttle is designed for versatility AND safety, so covering airborne transport certifications had to be addressed early on in our processes.
2022:
EpiGuard achieves ISO 13485:2016 certification validating our quality system and management.
Why is this important?
Safety and quality are non-negotiable in manufacturing of medical devices. ISO 13485:2016 provides a framework for meeting evolving regulatory and customer requirements.
2024:
Electrical safety tests with national deviations for US and Canada as per IEC60601-1 completed.
FDA 510k application in the works.